Previously, the nasal spray Covid-19 vaccine was approved by the China Food and Drug Administration for clinical trials in humans from August 27, 2020. However, up to now, the working group plans to launch a phase 1 clinical trial with 150 volunteers in the Hong Kong Special Administrative Region.
Technically, the nasal spray Covid-19 vaccine is a virulence reduction influenza virus vector vaccine, developed by inserting a mutant gene segment of the SARS-CoV-2 virus into a virulent reduced normal influenza virus vector force. In terms of mechanism of action, the modified influenza virus (vector) transports the genetic code (prickly proteins on the virus surface) for the antigen. These antigens will activate the body’s immune response.
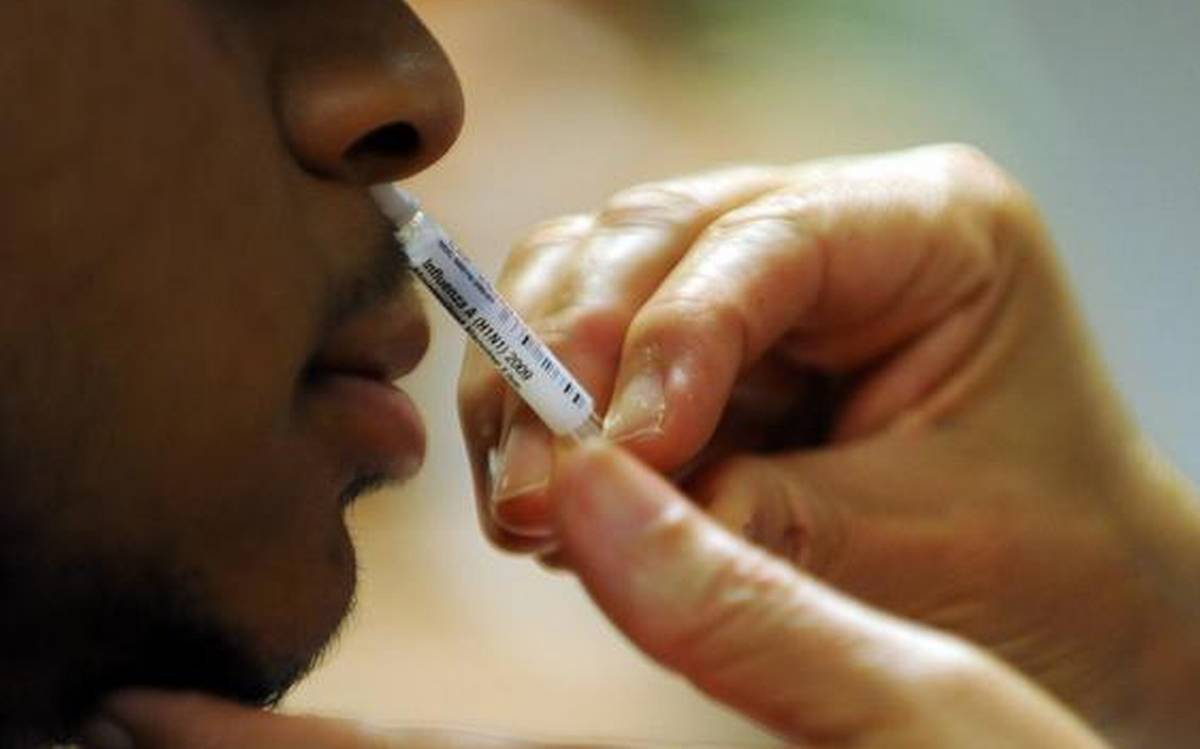
According to Mr. Tran Hong Lam – professor of Hong Kong University – the vaccine system of the University of Hong Kong researches based on influenza virus, which has the same properties as the nasal spray flu vaccine, has a very high safety, so it is likely to there is no obvious clinical manifestation.
Professor Sarah Gilbert, University of Oxford UK also affirmed that vaccination is not the only way to fight respiratory viruses. With a vaccine against this type of disease, the goal of the drug is to stimulate a strong immune system in the upper respiratory tract, then the lower respiratory tract, which are the places where the virus causes inflammation to the body, thus for SARS-CoV-2 is likely that the nasal spray vaccine will be most effective./.

